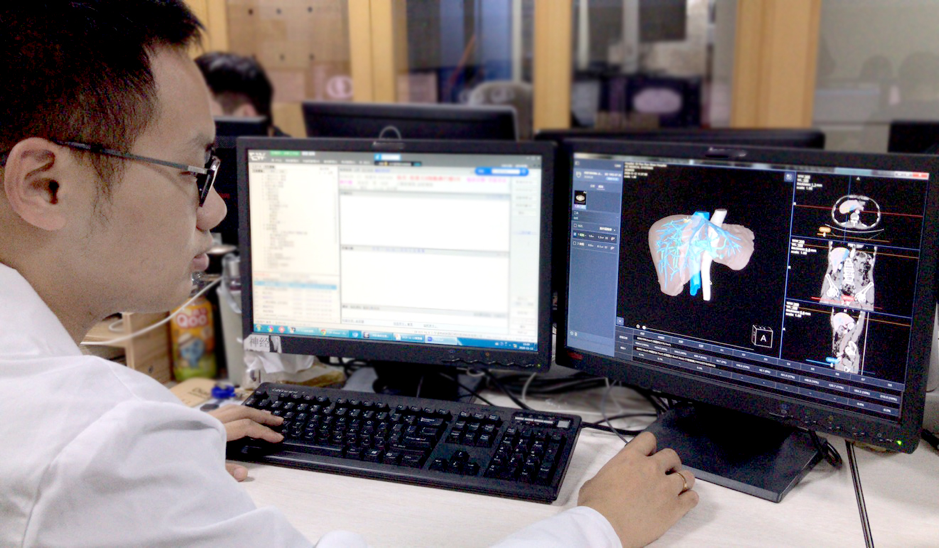
Chinese artificial intelligence pioneer SenseTime unveiled two AI-powered medical solutions for diseases related to the liver and the heart, as the company works hard to harness cutting-edge technologies to help doctors boost efficiency in diagnosis.
SenseTime said the two software solutions are respectively developed to enable faster AI-assisted analysis of Enhanced Liver Computed Tomography and Cardiac Computed Tomography Angiography. Cardiac CTA is a noninvasive test that uses special X-rays to focus on the coronary arteries.
The solutions can help doctors rapidly locate the focus of diseases and provide rich qualitative and quantitative analysis, SenseTime said.
The two solutions are part of applications for SenseTime's SenseCare® Smart Health Platform – an AI-powered platform that offers an array of healthcare applications for over 13 organs and body parts, covering the chest, cardiovascular system, the liver, pathology, orthopedics and radiotherapy.
The platform can assist doctors throughout the clinical process, from diagnosis and 3D surgery planning to rehabilitation tracking to meet the demands of various departments, including radiology, pathology, cardiology, orthopedics, surgery and radiotherapy, SenseTime said on its official website.
Jin Zhengyu, director of the department of radiology at Peking Union Medical College Hospital, a renowned hospital in China, said the two solutions developed by SenseTime reflect the inevitable trend of cooperation between industry and hospitals in research and application. They are expected to help doctors solve many clinical problems.
The latest products came shortly after SenseTime's AI-empowered software solution SenseCare-Lung Pro, which has been awarded the CE mark to meet the safety and performance requirements demanded in the European Economic Area.
The CE marking (an acronym for the French "Conformite Europeenne") certifies that a product has met EU health, safety, and environmental requirements, which ensure consumer safety.
The software enables faster and more automated diagnosis of lung diseases, including COVID-19 infections, based on CT image analysis.

